
The bronze crown shown here was found in the Judean desert. It is more than 5000 years old. Already then, people knew how to generate very high temperatures, hot enough to melt, refine, and alloy metals.
Understanding why solids melt is a more recent story. Lindemann’s theory of melting came out in 1910, 5000 years down the road. Only in 1985, Frenken and van der Veen showed that melting actually starts at the surface of the solid. So despite the thousands of years that have passed, significant advances in our understanding melting are fairly recent. For many years, my work in this area involved computer simulations of surface melting, done in collaboration with Joan Adler and Steve Lipson.
The list of students who worked on the Melting problem includes Amit Kanigel, Slava Sorkin, Shahar Levy, Pavel Bavli, Almog Danzig and Ori Scaly.
Recently, I decided to start an experimental program in this area. The questions I am trying to answer are at what temperature does the surface start to melt, and is the surface actually fluid once this process begins.
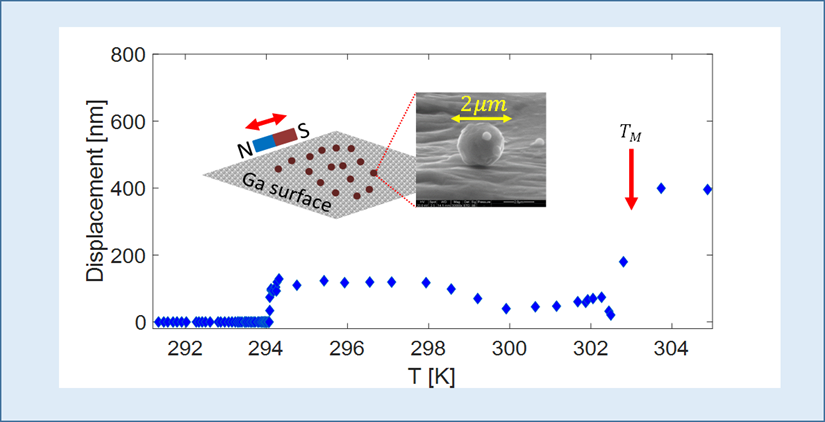
The first experiments in this area were part of the thesis work of Almog Danzig and Ori Scaly. They looked at the mobility of the surface of solid Ga as a function of temperature. They used small Iron particles dispersed on the surface as trackers. These particles were acted upon by a small moving magnet. The plot shows that the iron particles become mobile once the surface becomes fluid, at a temperature about 10K lower than the melting point TM . This seems a good way to detect the onset of surface melting.
The method Almog used could not be applied to study metals melting at temperatures higher than room temperature. In our current work, Steve Lipson and I use a non-contact optical method to look at the temporal self-correlation at different points on the surface. The non-contact method allows us to work with metals which melt at high temperatures. The method seems very sensitive and we have great expectations for the future.

