Hydra Morphogenesis
Morphogenesis, the emergence of well-defined patterns of functional tissues during development, is carried out by the collective dynamics of multiple biophysical and biochemical processes. These interconnected processes span all levels of organization, from local molecular events to large-scale biochemical and mechanical fields. One of the main challenges in morphogenesis research is to understand how all these dynamic processes integrate across scales, to form robust spatio-temporal developmental patterns that lead to a viable organism under variable environmental and internal conditions. Our research aims to shed light on the process of morphogenesis from a biophysical perspective, focusing on the role of mechanical forces and feedback in the formation and stabilization of the body plan.
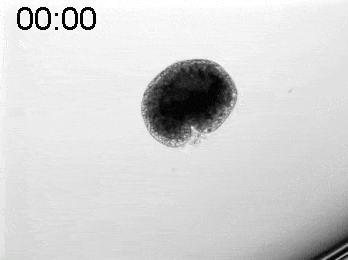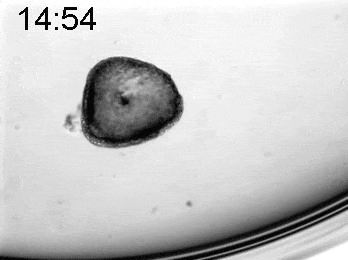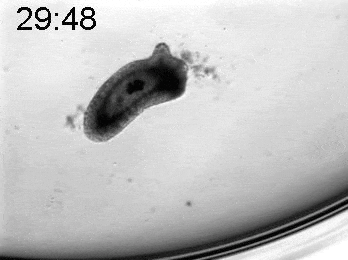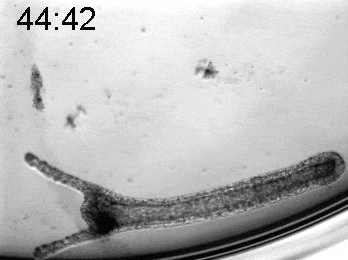
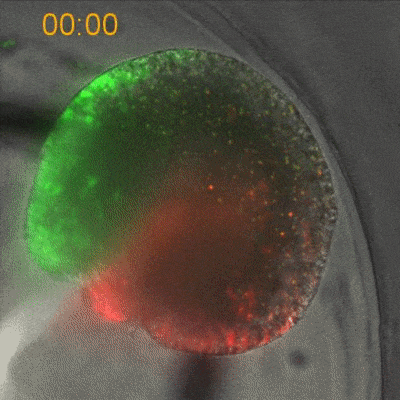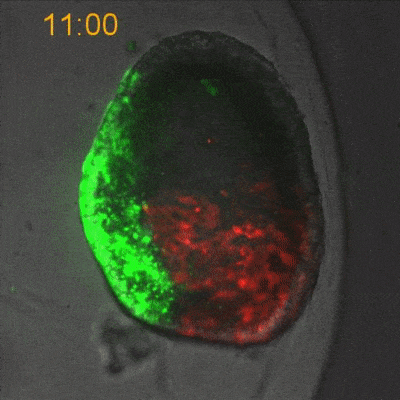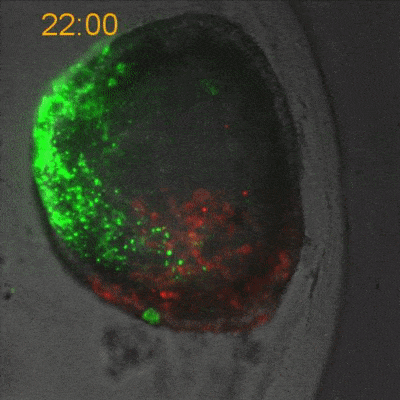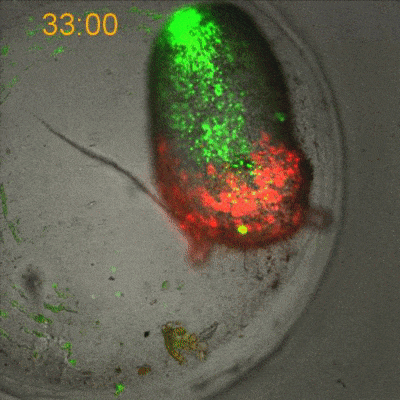
To explore the mechanical basis of morphogenesis, it is beneficial to seek the simplest and most flexible experimental model system. To that end, we work on Hydra, a small fresh-water predatory animal. Hydra is a multicellular animal with ~105 cells that has a uniaxial body plan and exhibits remarkable regeneration capabilities. Thanks to its simple body plan and experimental flexibility, Hydra became a classic model system for morphogenesis studies. Indeed, historically, research on Hydra regeneration inspired the development of many of the fundamental concepts on the biochemical basis of morphogenesis, including the role of an “organizer”, the idea of pattern formation by reaction-diffusion dynamics of morphogens, and the concept of positional information. In our research, we return to this classic model system, equipped with modern imaging and manipulation tools, and focus on its regeneration process as a powerful experimental platform to study the role of mechanics in morphogenesis. We aim to develop a phenomenological description of the mechanical mechanisms underlying the formation and stabilization of the body plan during Hydra regeneration at the level of cells and tissues.
This work is funded by an ERC consolidator grant (2019-2025)
Our current efforts are directed along the following research directions:
- Cytoskeletal dynamics
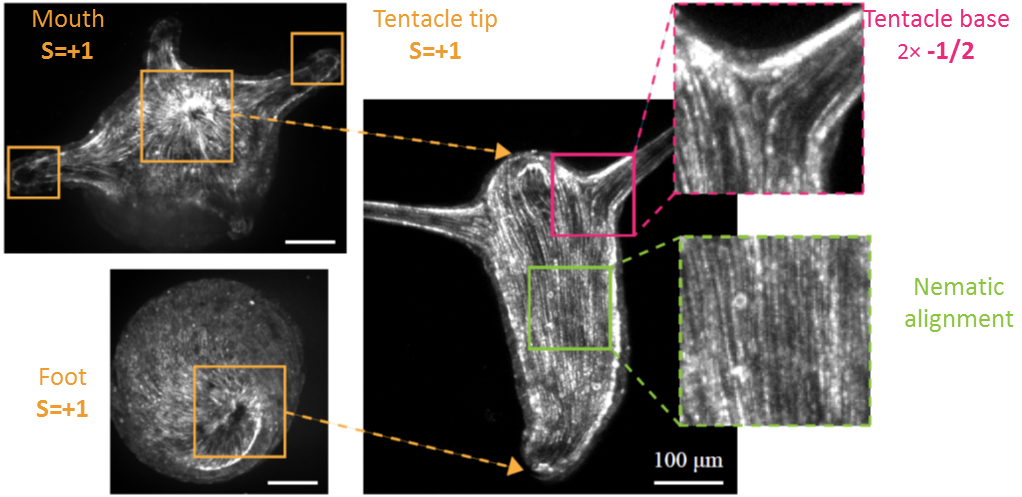
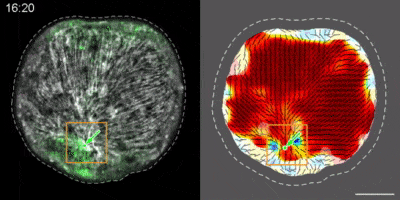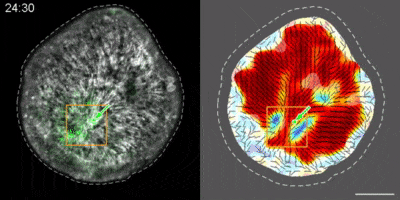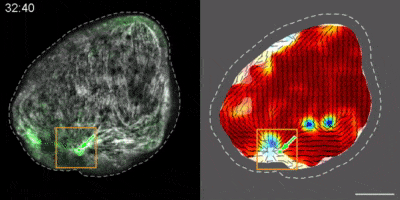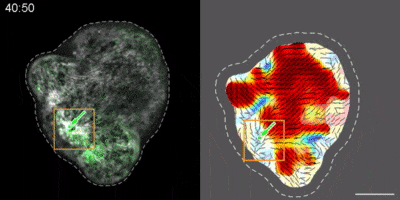
Hydra contains supra-cellular actin fibers aligned parallel to its axis in the ectoderm and in a circumferential orientation in the endoderm. This muscle fiber organization enables mature Hydra to contract and expand. From the physics point of view, the actin fibers exhibit a nematic order—large-scale arrays of aligned fibers— and its stable maintenance is essential for the body-plan robustness and stability. We have shown that the large-scale structural inheritance of the cytoskeletal organization and mechanical self-organization can direct the body axis in regenerating Hydra (Cell Reports, 2017).
We are currently studying actin fiber organization in regenerating Hydra as the collective dynamics of an active nematic system on a deformable spheroid. We hope that such a phenomenological description of actin fiber organization as an active nematic, will inspire the development of predictive effective theories based on active matter physics that will be able to predict the dynamics of nematic defects in regenerating Hydra and relate them to the patterning of the body plan (bioRxiv, 2020).
- Mechanical feedback
Our central hypothesis is that Hydra possess mechanical feedback mechanisms that are responsible for the large-scale coordination of cytoskeletal organization and cellular dynamics that leads to robust tissue organization, axis formation and regeneration. To directly probe the influence of mechanical forces and feedback on Hydra regeneration process we apply various external mechanical perturbations and study their influence on the regeneration process and the final morphology of the regenerated animal.
- Polarity
The question of how polarity of the body axis is established and maintained during morphogenesis is one of the central problems in developmental biology. Polarity requires global coordination across the entire organism to generate a well-defined body axis, and at the same time is also manifested locally in the behavior and differentiation of individual cells within the tissue. We seek to examine the contribution of mechanics and the dynamic organization of the actin cytoskeleton to the establishment and maintenance of polarity during regeneration. This will be combined with studies of the dynamics of bio-signaling molecules and the generation of their polar distribution, to provide insight into mechanisms that coordinate cell behaviors and establish body-axis polarity during morphogenesis.
Cell Mechanics
We are interested in understanding how large-scale cellular behavior emerges from the numerous underlying molecular events. We combine experimental work on relatively simple model systems, namely reconstituted actomyosin networks and keratocyte cells and fragments, with mathematical modeling, toward the goal of reaching quantitative understanding of cellular-scale dynamics and mechanics.
This work is funded by grants from the BSF (2008-2022), ISF (2015-2019) and an ERC starting grant (2008-2013)
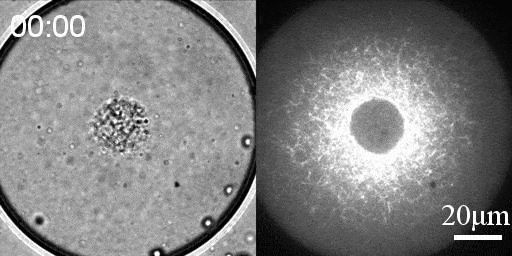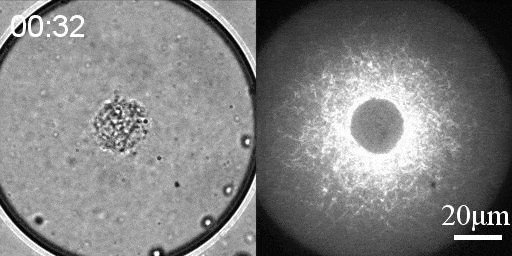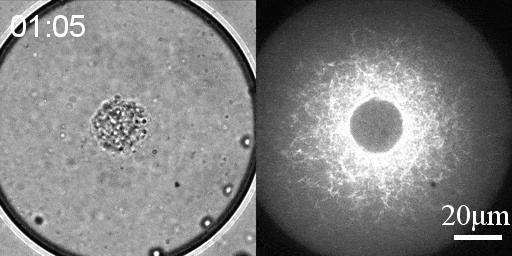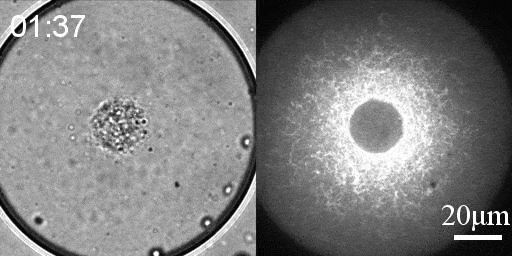
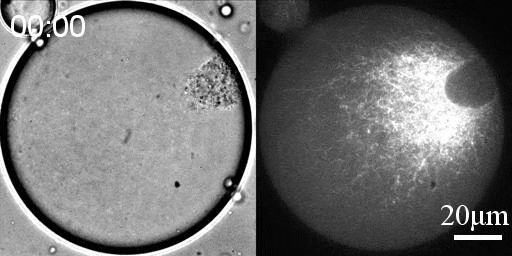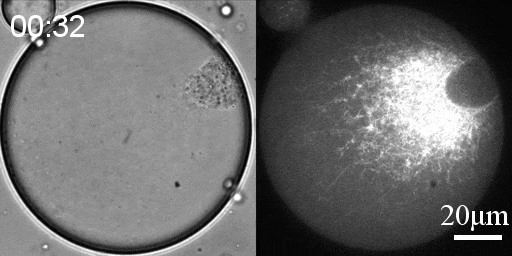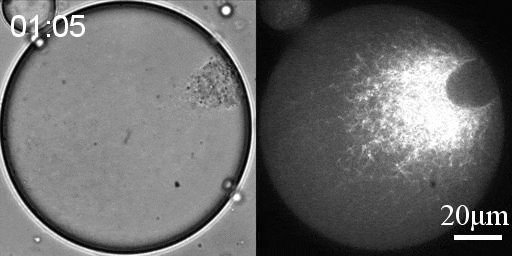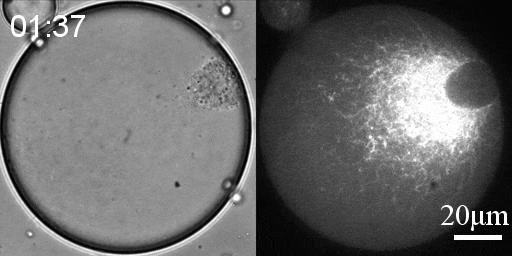
Reconstituted actomyosin networks in artificial cells
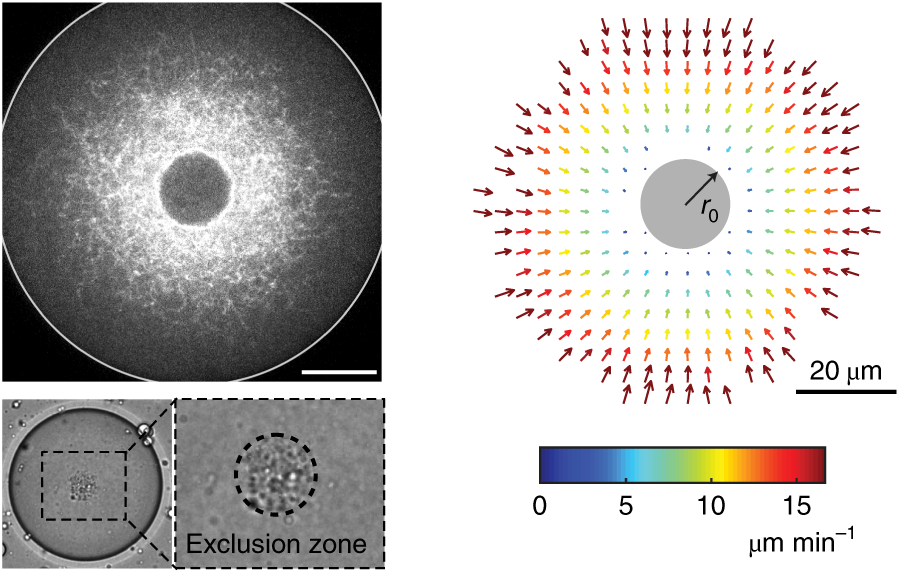
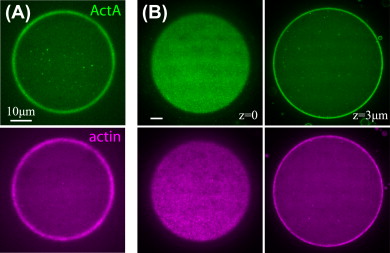
We developed a reconstitution approach to study the dynamic of actomyosin networks with rapid turnover in artificial cells. We are using this reconstituted system to study the dynamic behavior of contractile actomyosin networks in a controlled environment, with the long-term goal of developing artificial moving cells.
We developed a reconstitution approach to study the dynamic of actomyosin networks with rapid turnover in artificial cells. We are using this reconstituted system to study the dynamic behavior of contractile actomyosin networks in a controlled environment, with the long-term goal of developing artificial moving cells.
Using this system, we have been able to:
- Characterize the non-equilibrium phase transition that leads to cortical symmetry breaking through an abrupt transition into a state of global contraction (Elife 2014, Science Advances 2018)
- Discover a scaling relation between the active stress and network viscosity that leads to density independent contraction (Nature Physics 2019)
- Discover novel mechanisms for cellular centering and symmetry breaking, which are based on persistent contractile actomyosin flows and their hydrodynamic interactions with the fluid cytosol (eLife 2020).
Actin-based cell motility
A central challenge in motility research is to quantitatively understand how numerous molecular building blocks self-organize to achieve coherent shape and movement on cellular scales. One of the classic examples of such self-organization is lamellipodial motility in which forward translocation is driven by a treadmilling actin network. We use keratocyte cells and lamellipodial fragments, which are arguably the simplest natural model systems for lamellipodial motility, to study the basic biophysical aspects of the motility process. This research has led to significant contributions towards the quantitative understanding of actin-based lamellipodial motility. Specifically, we performed biophysical measurements of different aspects of motile cell behavior, including the tension in the cell membrane (Current Biology 2013, Biophysical Journal 2015) and the intracellular fluid flow (Nature Cell Biology 2009). These measurements, together with quantitative analysis of actin turnover (Current Biology 2017) and the dynamics of overall cell morphology and movement, led us (in collaboration with the group of Prof. Alex Mogilner, NYU) to the development of an integrated model of the mechanism of shape determination in motile cells (PNAS 2011, Current Biology 2017 ).